Abstract
Introduction: Although treatment advances have extended survival outcomes for patients with RRMM, nearly all patients eventually relapse. As potentially transformative treatment options emerge, understanding their impact on patient-reported outcomes in addition to clinical outcomes will be essential in evaluating their value in the therapeutic landscape. In particular, characterizing the potential impact of chimeric antigen receptor (CAR) T cell therapies for patients who have been triple-class exposed (TCE) to an immunomodulatory drug, proteasome inhibitor, and an anti-CD38 antibody will be important. This study aimed to establish a quantifiable depiction of HRQoL outcomes in TCE patients who may be eligible but have not yet received subsequent CAR T cell therapy.
Methods: This was a retrospective medical chart review combined with a single time point cross-sectional patient questionnaire conducted across 4 cancer centers in the US. All patients were 2-11 months post triple-class exposure with a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody. Patient demographics, clinical characteristics, treatments received, disease status, comorbidities, and symptoms/side effects were captured via an electronic case report form completed by the study sites. Patients completed a questionnaire providing demographic information and responses to the EQ-5D 5-level version (EQ-5D-5L) and visual analog scale (EQ-5D VAS), and the EORTC Core QoL Questionnaire (EORTC QLQ-C30) and Multiple Myeloma module (EORTC QLQ-MY20). Patient-reported outcomes were analyzed descriptively and evaluated alongside findings from the general population when available.
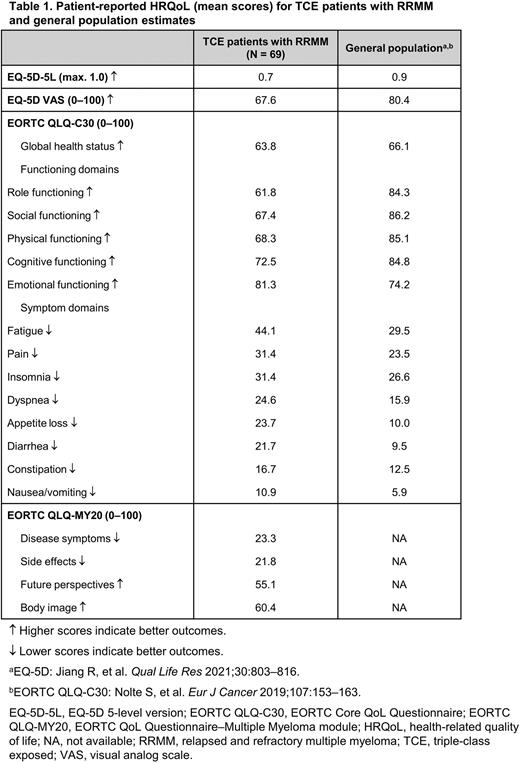
Results: A total of 69 patients were enrolled from April 21, 2021 to June 2, 2022. Mean age was 67 years (interquartile range, 62-72); 51% were women. Mean overall HRQoL scores were lower for TCE patients compared to the general population (Jiang R, et al. Qual Life Res 2021;30:803-816) on the EQ-5D-5L (0.7 vs 0.9/1.0, respectively) and EQ-5D VAS (67.6 vs 80.4/100), where higher scores indicate better QoL (Table 1).
On the EORTC QLQ-C30, the mean global health status score showed worse HRQoL for TCE patients than the general population (63.8 vs 66.1/100) (Nolte S, et al. Eur J Cancer 2019;107:153-163). Mean EORTC QLQ-C30 functioning domain scores, where higher scores indicate better outcomes, were worst for TCE patients in terms of role (61.8/100) and social (67.4/100) functioning. All functioning domain scores were worse for TCE patients than the general population, except for emotional functioning (Table 1). Mean EORTC QLQ-C30 symptom domain scores, where lower scores indicate better outcomes, were worst for fatigue and pain, and worse for TCE patients than the general population for fatigue, pain, insomnia, dyspnea, appetite loss, diarrhea, constipation, and nausea/vomiting (Table 1).
On the EORTC QLQ-MY20, mean scores for TCE patients were 23.3/100 for disease symptomatology and 21.8/100 for treatment side effects, where lower scores indicate better outcomes (Table 1). Mean scores for the EORTC QLQ-MY20 domains of body image and future perspective (measuring concerns about outlook and future health), where higher scores indicate better outcomes, were 60.4/100 and 55.1/100, respectively. General population estimates were not available for the EORTC QLQ-MY20.
Conclusions: This study demonstrates poor overall HRQoL among TCE patients with RRMM that was worse than the general population for nearly all measured domains. Fatigue, pain, and multiple aspects of functionality were particularly poor for these patients. These findings provide tangible patient-reported assessments of HRQoL following triple-class exposure prior to receiving subsequent therapy, highlighting the unmet need and quantifying the potential for improvement in HRQoL by more effective subsequent treatment options. These findings may also help clinicians working with TCE patients to navigate supportive care.
Disclosures
Shah:Oncopeptides: Consultancy; Indapta Therapeutics: Consultancy; GSK: Consultancy; Precision Biosciences: Research Funding; Allogene: Consultancy; CareDx: Consultancy; BMS/Celgene: Consultancy, Research Funding; Amgen: Consultancy; AstraZeneca: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Bluebird Bio: Research Funding; Janssen: Research Funding; TeneoBio: Research Funding; Poseida: Research Funding; Karyopharm: Consultancy; Kite: Consultancy; Nektar: Research Funding; CSL Behring: Consultancy; Sanofi: Consultancy; Sutro Biopharma: Research Funding. Vij:BMS, Takeda, Sanofi: Research Funding; BMS, Sanofi, Takeda, Janssen, GSK, Oncopeptides, Pfizer, Legend, Adaptive: Consultancy; Takeda, Bristol Myers Squibb, Sanofi, GlaxoSmithKline, Janssen, Oncopeptides, Harpoon, Adaptive, Legend, BeiGene, CareDx: Honoraria. Silbermann:Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Hillengass:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Beigene: Honoraria; Beijing Life Oasis Public Service Center: Honoraria; Amgen: Honoraria; GSK: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Skyline: Membership on an entity's Board of Directors or advisory committees; Axxess Network: Membership on an entity's Board of Directors or advisory committees; Curio Science: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; Beijing Medical Award Foundation: Honoraria; Adaptive: Honoraria. Dhanasiri:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Braverman:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Dhanda:BMS: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal